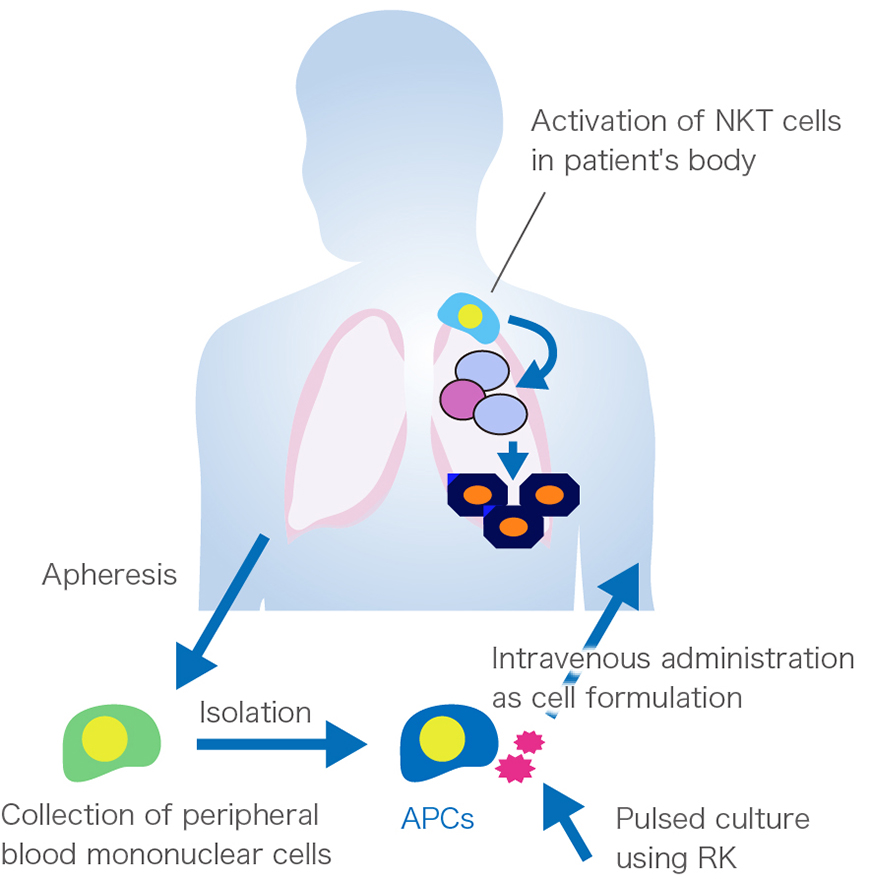
To manufacture RK-pulsed APCs, autologous APCs are cultured with a novel NKT ligand ”RK” that activates NKT cell much stronger than the conventional NKT ligand ”α-galactosylceramide”.
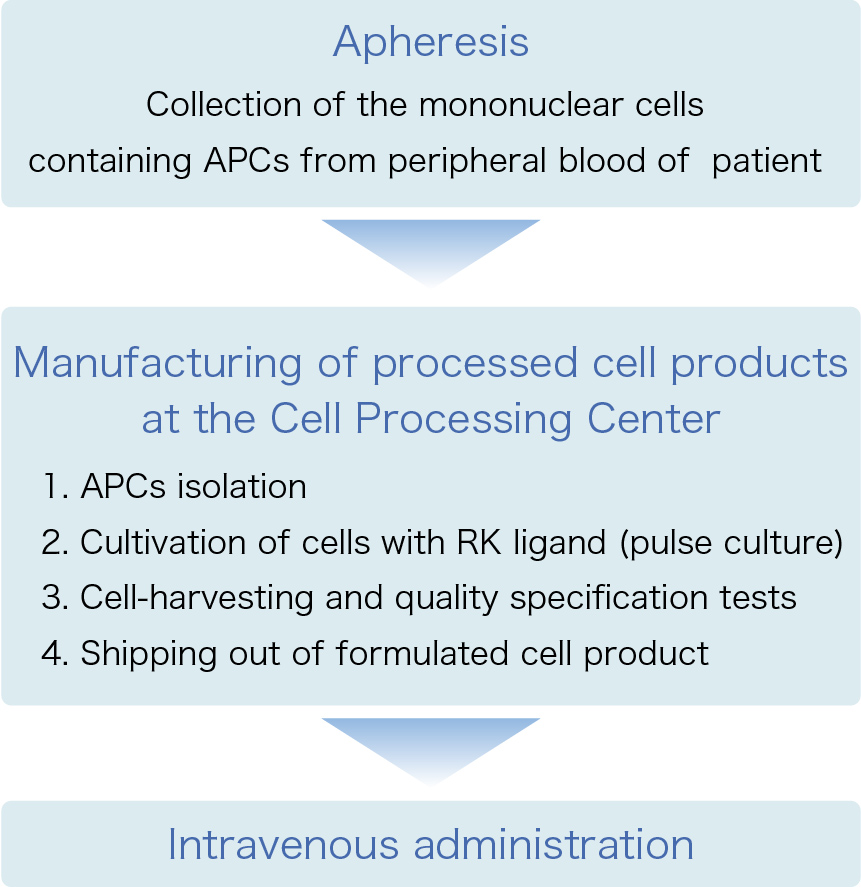
Firstly, apheresis is carried out with the patients to collect mononuclear cells containing APCs from their peripheral blood. Then, the cells are processed at the Cell Processing Center.
In more detailed terms, APCs are initially isolated from blood, followed by cultivation with the novel ligand “RK” for a short period. RK-pulsed autologous APCs are then harvested, and, after testing to ensure that they meet quality specifications, they are formulated properly and transported to medical facilities as frozen products. The aim of this treatment is to energize the patient's immune system through first activation of NKT cells via the administered NKT ligand-pulsed autologous APCs, and finally eliminate cancer cells by fully mobilizing the immune system.
The cell product consisting of RK-pulsed autologous APC is used as regenerative medicine that is produced using patients' own cells. Development of this cell product is a lead program at Ambicion, Inc. and occupied a central product pipeline. The Japan Agency for Medical Research and Development has selected this research program (“NKT cell-targeted anti-cancer treatment using a novel NKT ligand, RK”) as Seeds B in the Translational Research Strategic Promotion Program, based at Keio University, and its progress is currently being made with collaborative development by Riken and Keio University with the aim of initiating a Phase I clinical study in early 2018.
Production methods and treatment processes